Richard Harris
Stories
-

Fauci to step down in December after decades of public service
Dr. Anthony Fauci helped guide the U.S. through the COVID crisis, and earlier in his career played a key role in the response to AIDS. He has served under seven presidents.
-

On the 25th anniversary of 'Tuesdays with Morrie,' the teaching goes on
Publisher after publisher rejected the memoir before Doubleday took a chance in 1997. At its core, the book is about the power of relationship and focusing on others.
-
Scientist Luc Montagnier, who discovered the virus that causes AIDS, is dead at 89
Luc Montagnier, the scientist who discovered the virus that causes AIDS, has died at 89. His key contribution came at a time when AIDS was mysterious and uniformly deadly.
-
Amateur Mushroom Photographer Makes A Big Discovery
A mushroom thought extinct in the US for 100 years has been rediscovered. It's an example of the remarkable synergy between amateur and professional fungus aficionados.
-
In Kids, The Risk Of COVID-19 And The Flu Are Similar — But The Risk Perception Isn't
The risk of serious COVID-19 illness in children is comparable to their risk from the flu, but many parents seem more concerned about coronavirus. The issue of risk perception has a lot do with it.
-
'Monoclonal Antibodies' Can Keep Coronavirus In Check, But Won't Stem Michigan Surge
Drugs that can help keep COVID-19 patients out of the hospital are playing only a small role in Michigan, where the pandemic is accelerating. Logistical challenges are to blame.
-

Drugs Targeting Immune Response To COVID-19 Show Promise
Researchers are reporting some progress in their search for drugs that tamp down the overwhelming immune reaction that can kill a patient with COVID-19.
-
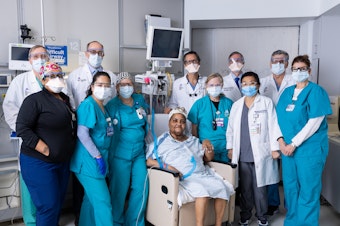
Woman Gets New Windpipe In Groundbreaking Transplant Surgery
A medical team in New York City says it has performed the first complete surgical transplant of a trachea. These kinds of transplants are one of the last big transplant challenges.
-
AstraZeneca's Latest Report Supports Effectiveness Of COVID-19 Vaccine
AstraZeneca has updated the data about its COVID-19 vaccine after criticism that its previous statement was based on incomplete information. The update still finds that the shot is safe and effective.
-
Antibiotic Use Ran High In Early Days Of COVID-19, Despite Viral Cause
Many doctors have used antibiotics to treat COVID-19 patients, but that's largely unnecessary — and could even promote drug-resistant germs.