FDA says watch out for fake Ozempic, a diabetes drug used by many for weight loss
Thousands of counterfeit units of Ozempic injections have been discovered in the legitimate supply chain and seized, the Food and Drug Administration says, but some fake products may still be in circulation.
Novo Nordisk, the Danish pharmaceutical company that manufactures Ozempic, which has become wildly popular for weight loss, is working alongside the FDA to test the seized drugs, but have yet to announce any quality or safety findings, according to a Thursday news release.
The FDA said it's "aware of five adverse events from this lot, none of which are serious and are consistent with known common adverse reactions to authentic Ozempic, which are nausea, vomiting, diarrhea, abdominal pain and constipation."
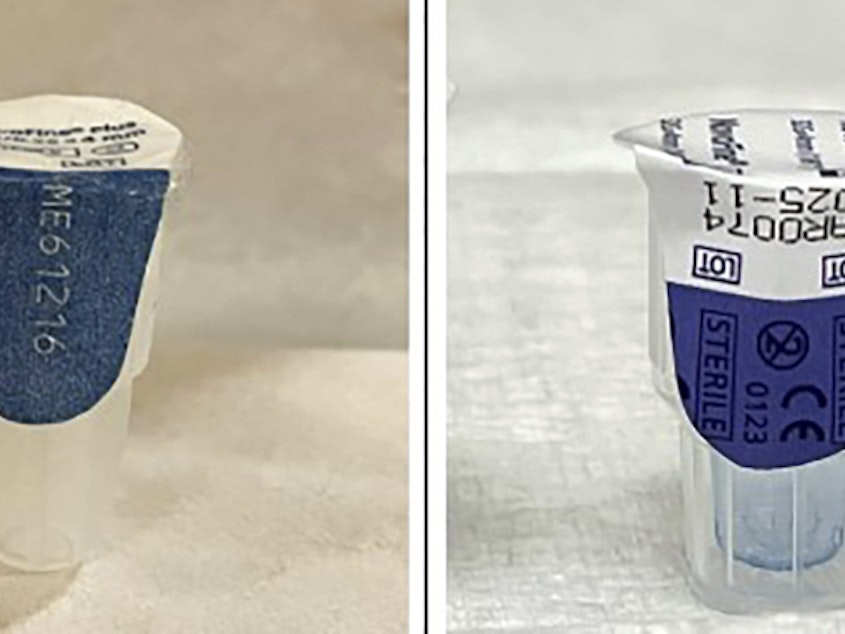
The FDA is warning wholesalers, pharmacies, health care practitioners and patients alike to check their product labels for counterfeit units and report questionable products to the agency. Fakes are labeled with lot number NAR0074 and serial number 430834149057, it says.
Even the needles in the fake units are counterfeit. The FDA says it cannot confirm whether the needles are sterile or not, which poses an increased risk of infection for consumers.
Sponsored
Photos on the FDA news release show examples of authentic and counterfeit products so consumers can spot the fakes.
Novo Nordisk reported over $24.5 billion in sales in the first nine months of 2023, and more than one third of that is attributed to Ozempic. The weekly injection is approved for lowering blood sugar levels in patients with Type 2 diabetes. However, its increase in popularity is credited to its ability to quell food cravings via its active ingredient, semaglutide, according to the University of California-Davis.
The FDA has not approved Ozempic for weight loss, but another Novo Nordisk drug utilizing semaglutide, Wegovy, was approved in 2021 for weight management. [Copyright 2023 NPR]



